|
|
|
 |
In recent months, two peer-reviewed publications about the Episealer® have been published, including well over 100 patients in total. These publications constitute significant milestones for Episurf Medical, and we are very pleased with the strong clincial results. Despite challenging market conditions during this pandemic, we have continued to see an increase in the use of the Epiealer Knee implants. We have also performed additional surgeries with the new implant, the Episealer Talus. The first surgeries in the US and Europe within our US IDE clinical trial have been performed in recent months, and we look forward to more activity in this important clinical trial. In this Newsletter, you will find highlights from Episurf Medical, and I would especially encourage you to read the interview with Dr Sadlik, the first surgeon in Poland to perform an Episealer Talus surgery.
Tomorrow, Thursday December 10, we are running webinars with Mr Tim Spalding, Dr Johannes Holz, Prof. Martin Lind, and Episurf founder Prof. Leif Ryd. The webinars will focus on clinical results, the role of the Episealer in the treatment algorithm, and surgical technique. You are very welcome to attend, and you will find all relevant information in this Newsletter.
Pål Ryfors
Chief Executive Officer, Episurf Medical
|
|
|
|
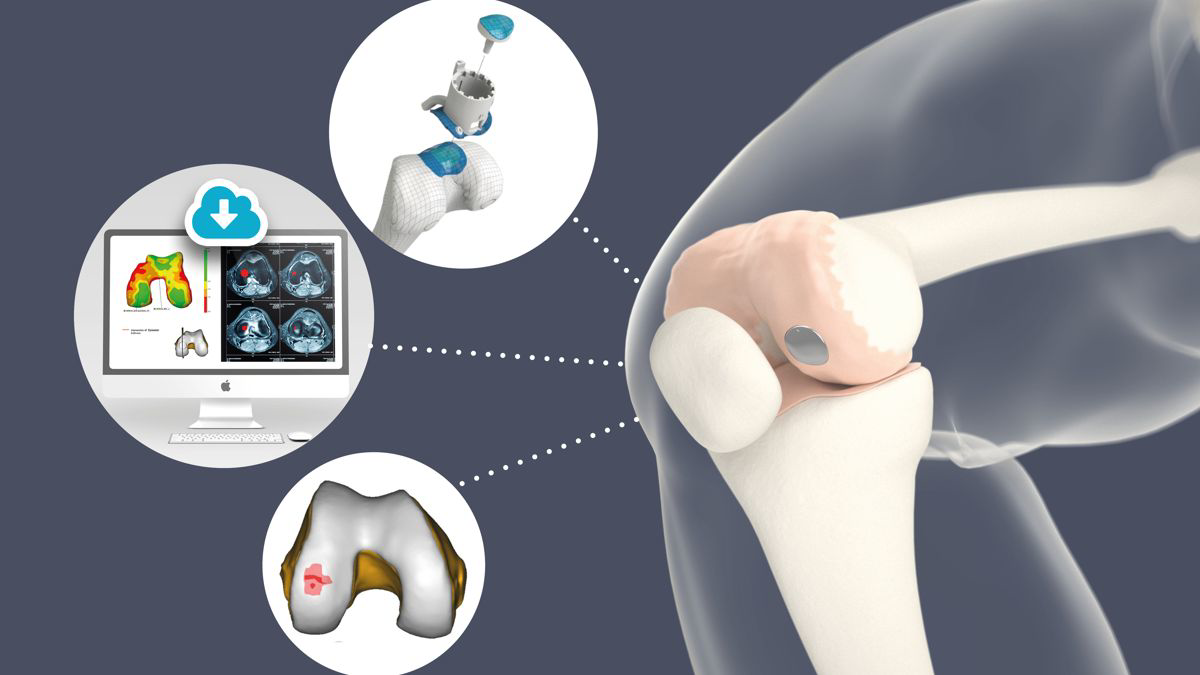
Since the very beginning, follow up of the clinical outcome for Episealer patients has been of highest importance for Episurf. Thanks to that, we have in this quarter been able to announce the publication of two peer-reviewed manuscripts with the results from two independent multicentre studies.
|
|
The first study is a 24 monthts' follow up of 80 patients from 9 clinics in 6 European countries.
The study has been published in KSSTA and concludes that the Episealer Knee implants are safe with a low failure rate of 2.5% and result in clinically significant improvement.
It also indicates a definitive place for the Episealer Knee implant in the management of focal chondral or osteochondral defects.
|
|
The second study, with 30 of the first Swedish Episealer Knee patients, has been published in Cartilage.
This study highlights that the Episealer Knee implant is not only a short term solution providing significant clinical improvement but also showing a very low revision rate of 3.3% at a mean of 55 months.
|
|
|
|
Please join our webinar, where three members of the study group behind the recent publication "Patient‑specific metal implants for focal chondral and osteochondral lesions in the knee; excellent clinical results at 2 years" present their experience of the Episealer technology.
|
|
|
|
|
 |
-Dr Sadlik, you performed the first Episealer Talus procedure in Poland at a live surgery last month, what convinced you to start using the Episealer Talus implant?
As I have been dealing with ankle damage for many years, my awareness of the biomechanical requirements of this joint, which is the most heavily loaded human joint per cm2 surface, has inspired my confidence in this concept. Professor Niek van Dijk informed me about this individually designed implant for the partial resurfacing procedure and offered to help me with the first implantation of the Episealer Talus implant, which encouraged me a lot. Just like Professor Niek van Dijk, I believe that the key to success is to block leakage of the synovial fluid into the ankle bone and to perfectly recreate the shape of the surface.
-What made you suggest an Episealer Talus implant for this specific patient?
In my practice, I use technologies of biological transplants to repair cartilage defects of the ankle joint. I avoid the OATS technique because it does not perfectly recreate the shape of the articular surface and requires sacrificing the healthy cartilage surface of a healthy knee joint. I usually use the BIOR (Biological Inlay Osteochondral Reconstruction) technique, which is derived from the well-known "Sandwich" technique developed in the 1990s. The patient qualified for this surgery was treated by me using the BIOR technique. Despite the lapse of 14 months after the surgery, the lady still felt pain that prevented her from standing and walking for longer periods. The MRI examination showed that the biological graft was partially incorporated, but about 50% of its volume was necrotised. As new subchondral cysts were forming, the Episealer implant seemed to be the perfect solution. To my surprise, 7 weeks after the Episealer implantation, the patient began to walk without crutches and didn’t feel any pain in the ankle joint.
-For which patients do you plan to use the Episealer Talus implant?
In my opinion, the best solution for cartilage-bone damage to joints is the regeneration of defects with the use of biological implants that can recreate living tissue at the site of damage to the joint surface or subchondral cyst. The talus has relatively little blood supply, therefore biological reconstruction is not always successful. Failures mainly affect people over 45 years of age or people with low biological potential. Also, patients with large subchondral cysts or after aggressive treatment with the Microfracture procedure do not have a good prognosis for treatment with biological implants. In these situations, Episealer seems to be the preferred choice, even though we do not know its survival rate yet.
-What benefits do you find with Episealer?
The Episealer implant allows perfect fit to the articular surface individually for each patient. The patient may put stress on the ankle relatively soon. The system of individual printed guiding devices protects the surgeon against technical errors. The implantation technique is logical and relatively simple. However, as always, we will have to wait for a reliable evaluation of the sustainable results of this promising new technology.
|
|
|
| | |
The first Episealer surgery in the US was performed in October by Dr Doug Haltom, MD at the West Tennessee Bone & Joint Clinic in Jackson, Tennessee (Clinical Research Solutions, Jackson, TN). The surgery was performed within Episurf's IDE Clinical Trial, EPIC-Knee: Episealer Knee System IDE Clinical Study.
|
The first Episealer surgery in Scotland was performed at BMI Albyn Hospital in Aberdeen, Scotland by Orthopaedic Surgeon Mr Bidwell and his team.
|
 | |
The first Episealer surgery in the US was performed in October by Dr Doug Haltom, MD at the West Tennessee Bone & Joint Clinic in Jackson, Tennessee (Clinical Research Solutions, Jackson, TN). The surgery was performed within Episurf's IDE Clinical Trial, EPIC-Knee: Episealer Knee System IDE Clinical Study.
|  | |
The first Episealer surgery in Scotland was performed at BMI Albyn Hospital in Aberdeen, Scotland by Orthopaedic Surgeon Mr Bidwell and his team.
|
|
| | |
Dr Sotowski and his team from Warsaw performed the first Episealer surgery in Poland.
|
The first Episealer surgery in Asia was performed in October, at a Hong Kong clinic.
|
 | |
Dr Sotowski and his team from Warsaw performed the first Episealer surgery in Poland.
|  | |
The first Episealer surgery in Asia was performed in October, at a Hong Kong clinic.
|
|
|
|
| | |
The first Episealer Talus surgery in Scandinavia was performed at the Arcademy Clinic, at Sophiahemmet, Stockholm, Sweden. The surgery was performed by Dr Jouko Kivioja and his team, supported by Prof. Niek van Dijk from the FIFA Medical Center of Excellence, Madrid, Spain.
|
Dr Giulia Favilli, Foot and Ankle Specialist at Casa du Cura Porta Sole, Perugia performed the first Episealer Talus case in Italy, assisted by Prof. Niek van Dijk.
|
 | |
The first Episealer Talus surgery in Scandinavia was performed at the Arcademy Clinic, at Sophiahemmet, Stockholm, Sweden. The surgery was performed by Dr Jouko Kivioja and his team, supported by Prof. Niek van Dijk from the FIFA Medical Center of Excellence, Madrid, Spain.
|  | |
Dr Giulia Favilli, Foot and Ankle Specialist at Casa du Cura Porta Sole, Perugia performed the first Episealer Talus case in Italy, assisted by Prof. Niek van Dijk.
|
|
|
|
|
|
|
|
|
In recent weeks, Episurf Medical has published several relevant news. Please click on the links below to access the most relevant press releases:
|
|
|
|
|
|
If you are not yet a subscriber, you are very welcome to follow the links below to sign up for our press releases and/or newsletters:
|
|
|
|
|
|
|
|
|
|
|
Episurf Medical has a Privacy Policy in line with the European General Data Protection Regulation (GDPR). Read more about our policy here>>
If you no longer wish to receive our newsletter, please click the 'unsubscribe' link at the bottom of this newsletter.
|
|
|